The most simple, accurate, and reliable temperature monitoring system for vaccine storage and handling
Once Potency Is Lost, It’s Lost Forever
Vaccines are sensitive to temperature variance. That’s why the CDC and VFC programs have a long list of requirements to monitor them. You must monitor vaccine temperatures throughout every step of cold chain logistics. Otherwise, vaccines risk losing potency.
The CDC states that improper vaccine storage conditions and temperature variance are the number one cause of vaccine potency loss. According to the World Health Organization, vaccine storage facilities discard about 50% of inventory due to non-compliant storage and handling.
Dispensing ineffective vaccines threatens public safety, facility credibility, and individual careers. Receiving real-time alerts for temperature excursions through digital data loggers (DDLs) and thermometers can prevent asset loss and protect public safety. Choose a vaccine temperature monitoring system that automates meeting compliance. Never throw away expensive inventory again or threaten your facility’s reputation.
The SensoScientific Difference
Accurate, reliable environmental monitoring that makes meeting compliance the easiest part of your day.
24/7 Immediate Alert Notifications
You receive real-time alert notifications the moment your digital data logger measurements exceed an acceptable range. The alerts are sent via voice, email, text, SMS, pager, fax, and more!
Comprehensive Reporting
Meet compliance with every possible reporting option on the SensoScientific Validated Cloud, including automated reports in your email.
A2LA Accredited & NIST Traceable Calibration
Our in-house A2LA accredited Calibration Lab provides NIST traceable probes and is ISO/IEC 17025 certified.
Unlimited Cloud-Based Data Access
Our cloud platform is FDA 21 CFR Part 11 (ERES) compliant and stores unlimited data. Never fail an audit with SensoScientific.
Ensure Vaccine Potency & Protect Public Safety
Our vaccine temperature monitoring system is engineered to exceed vaccine monitoring requirements and automate meeting compliance. Our system complies with temperature monitoring guidelines found in CDC’s Vaccine Storage and Handling Toolkit and meets VFC program requirements in every state.
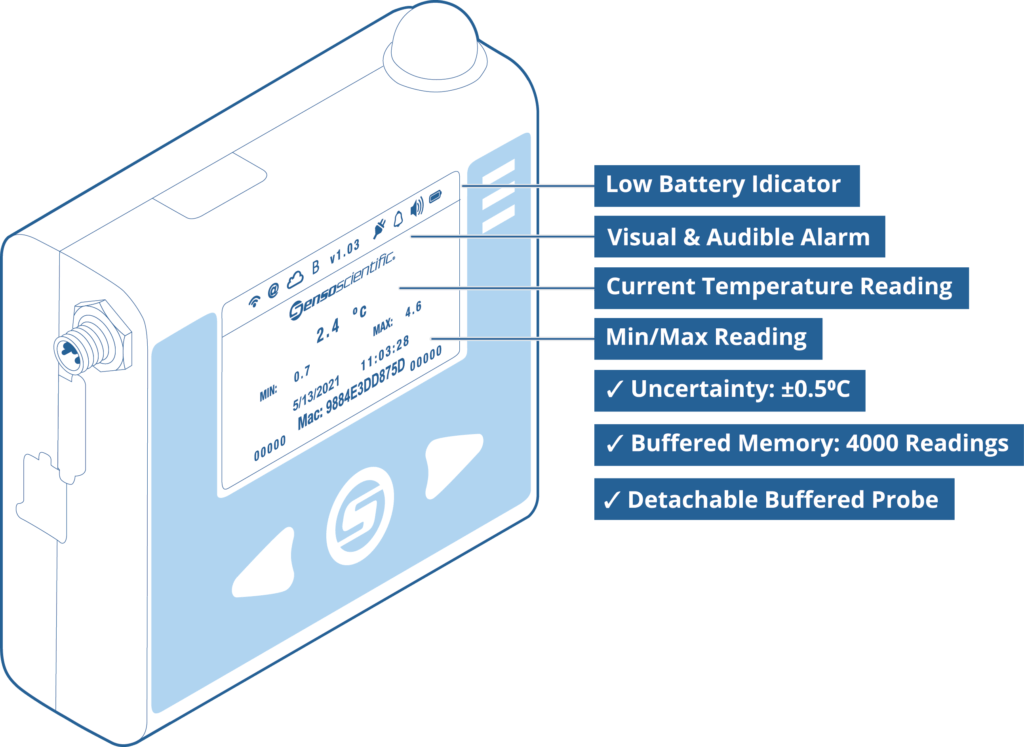
- Digital Display
- Detachable Probe in Thermal Buffer
- Current & Min/Max Display
- A2LA & NIST Calibration Certificates
- Accuracy ±0.5°C
- Immediate Alert Notifications
- Visual & Audible Alarm
- Low-Battery Indicator
- Custom Logging Intervals
- Buffered Memory: 4000 Readings
- Ok Button for Real-Time Readings
- No External Software Required
- Longest Warranty in the Market
- ISO/IEC 17025 Calibration Lab
Product Examples
Request Pricing and Information
"*" indicates required fields
Compliance Automation and Turnkey IoT Solutions
Meet compliance easily with SensoScientific. Our environmental and vaccine temperature monitoring system meets even the most stringent of regulatory requirements. Our clients include healthcare facilities, IDNs, pharmacies, universities, laboratories, and restaurants nationwide. From product development to installation and technical support, ours is an end-to-end solution that helps you meet and exceed compliance standards.





